By: Varsha Meghnani, Ph.D., NRCC, ASCP
The COVID-19 pandemic due to the SARS-CoV-2 virus has led to a global health emergency with more than 3 million deaths worldwide [1]. Due to the high transmission rate of the virus, it frequently passes from one host to another leading to lots of random mutations. These mutations affect the three key features of the virus (i.e., transmissibility, virulence, and response to the vaccines), which therefore makes it difficult to control [2].
It is very essential to break the chain and stop the spread of the virus, which will reduce the chance of further mutations. Wearing masks, social distancing and vaccinations are the commonly used ways to stop the spread.
Neutralizing antibodies generated as part of the host immune response can also impart protection against the virus as it blocks the entry of the virus into the cell and can indirectly minimize the spread.
MORE: What Exactly is a Neutralizing Antibody?
The virus uses the receptor-binding domain (RBD) of the spike protein for recognizing the human cell surface receptor, angiotensin-converting enzyme-2 (ACE2), and enter into the cell. As part of the immune response, the body creates antibodies and the fraction of the antibodies called neutralizing antibodies provide protection against future infections from viruses. Neutralizing antibodies can combat the viral pathogenesis by two major mechanisms: First, binding to the virus can prevent target cell binding and entry into the host cell. Secondly, antibody binding tags the infected cells for phagocytosis and eventually leads to the death of the infected cell [3].
Table 1. Neutralizing antibody activity [9].
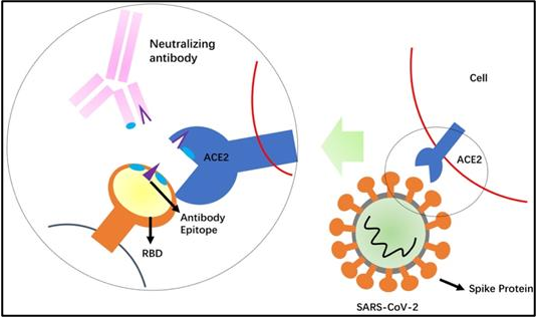
The neutralizing antibodies are generated in the body after exposure to the virus and/or after vaccination as part of the host’s own active immunity. When neutralizing antibodies are taken from the host and used for treating another COVID-19 patient, it is called passive immunity. The active immunity acts slow but is long-lasting, whereas the passive immunity is fast-acting but lasts for a shorter time duration.
Neutralizing antibody serological tests
Tests that detect and quantify neutralizing antibodies against SARS-CoV-2 are useful in assessing the infection rate, herd immunity, and in assessing vaccine efficacy. These tests can be a supplementary diagnostic tool when combined with the RT PCR-based molecular tests. The serological tests are also used to screen the therapeutic convalescent plasma for treating high-risk COVID patients. The FDA recommends only high titer COVID-19 convalescent plasma for the treatment of high-risk COVID-19 patients.
However, the variabilities in the performance of the serological test make their result interpretation difficult. Some of the common factors that lead to the performance variabilities are specimen type, specimen collection time, antigen used, antibody type tested, method sensitivity, and specificity. Harmonization is very crucial in minimizing the variabilities and getting consistent results [4]. The majority of these tests fall within two categories: qualitative, or semi-quantitative ELISA assays. Qualitative assays only provide the presence and absence of the type of antibody detected. Semi-quantitative assays provide more detailed information as they detect the range of the antibodies present and hence predict the level of immunization.
Table 2: FDA authorized tests to screen the High Titer COVID-19 antibodies for Therapeutic Convalescent Plasma [5].
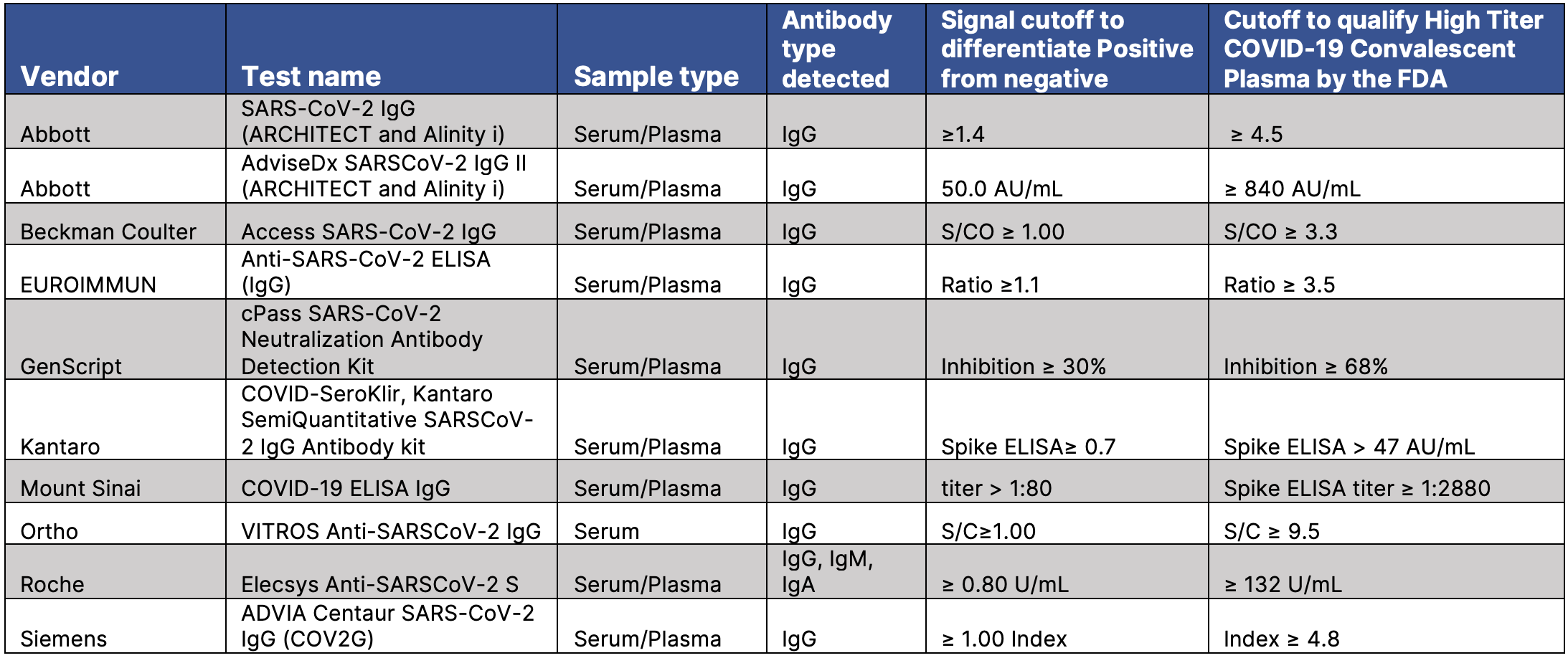
Individuals who have been vaccinated and/or recovered from a recent SARS Cov-2 infection should be tested to access the immunity level against the virus.
Therapeutic neutralizing antibodies
Neutralizing antibodies used for therapeutic purposes can either be polyclonal antibodies derived from convalescent patients or monoclonal recombinant antibodies derived in the lab. The convalescent plasma is the simplest and most direct approach to treating SARS-CoV-2. However, the approach has some technical challenges, which is why convalescent plasma therapy is still not considered the first-line therapy. The therapeutic effect of convalescent plasma on COVID-19 is determined by the level of SARS-CoV-2 neutralizing antibody titer (NAT). The neutralizing antibodies titer varies a lot among the donors. The level of neutralizing antibodies titer peaks around 12 weeks and then slowly wanes [6]. So, it is very important to screen the donors for the right amount of neutralizing antibodies titer prior to using it for therapeutic purposes.
The FDA issued an Emergency Use Authorization (EUA) for the use of high titer COVID-19 convalescent plasma, for the treatment of hospitalized patients with COVID-19, early in the course of the disease. The use of low titer COVID-19 convalescent plasma is not authorized under this EUA. In addition, some of the adverse reactions of convalescent plasma therapy are chills, fever, anaphylactic reactions, transfusion-related acute lung injury, circulatory overload, and hemolysis, etc. Therefore, it is crucial to understand how, when and for whom to use convalescent Plasma therapy for maximum therapeutic benefit. Also, the associated benefit-to-risk ratio should be assessed prior to considering the CP therapy. Convalescent plasma therapy is cheap and convenient, but should only be used in situations when other resources are not available [7].
Learn more: Antibody Testing Reimbursement
Monoclonal recombinant antibodies
Another neutralizing antibody therapy approach is monoclonal recombinant antibodies, which consist of steady titers of the neutralizing antibodies. Monoclonal antibodies are laboratory-made proteins that mimic the immune system’s ability to combat the SARS-CoV-2 virus. Bamlanivimab and etesevimab are the two therapeutic neutralizing antibodies approved by the FDA under the EUA. Both of these antibodies bind to spike protein and block spike protein attachment to the human ACE2 receptor. The EUA issued by the FDA permits the emergency use of bamlanivimab and etesevimab together for the treatment of mild to moderate COVID-19 symptoms in adults and pediatric patients with positive SARS-CoV-2. It also covers those who are at high risk for progressing to severe COVID-19 and/or hospitalization [8].
Takeaways
In conclusion, neutralizing antibodies used in combination with other medications, are an effective approach for both prophylactic and treatment. That being said, more investigation of neutralizing antibodies is required to optimize the therapy and determine the optimal dosing regimen. It is important to establish which at-risk individuals would benefit most from prophylactic neutralizing antibodies, the duration of protection offered by these antibodies, and any potential impact of antibody therapy on subsequent vaccination. It will also be important to determine the optimum timing for the administration of neutralizing antibodies on the basis of viral load, serology, and other potential clinical factors.
References
| [1] | “Worldometer COVID-19 Coronavirus Pandemic Updates.”. |
| [2] | V. Hackethal, “Tracking the Evolution of SARS-CoV-2 What do scientists know about its behavior, and how does that help predict what comes next?,” May 6, 2021. |
| [3] | P. C. Taylor, “Neutralizing monoclonal antibodies for treatment of COVID-19,” Nature Reviews Immunology , 2021. |
| [4] | K. A. K. Sofie Føns, “How can we interpret SARS-CoV-2 antibody test results?,” Pathogens and Disease, 2021. |
| [5] | C. P. E. L. o. Authorization, “Convalescent Plasma EUA Letter of Authorization,” The FDA, March 2021. |
| [6] | E. H. Y. Lau, “Neutralizing antibody titres in SARS-CoV-2 infections,” Nature Communications, 2021. |
| [7] | Q. Zhao, “Challenges of Convalescent Plasma Therapy on COVID-19,” J Clin Virol, 2020. |
| [8] | Lilly, “Emergency Use Authorization (EUA) for the Treatment of COVID-19 Bamlanivimab and etesevimab: Factsheet for Healthcare provider”. |
| [9] | G. Zhou, “Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2,” Int J Biol Sci. , 2020. |