Disclaimer: This article was updated on March 14, 2023, to ensure the information provided is up to date.
Are you thinking about opening a clinical laboratory? If so, and you’ve identified your test menu, instrumentation, and have hired a Laboratory Director, you’re now ready to obtain a CLIA certificate. “What are the next steps in the process to apply for a CLIA license?” is something you may ask. We are here to help!
Here is a brief overview of how to apply for your CLIA certificate:
First, download the CMS-116 Clinical Laboratory Improvement Amendments (CLIA) application form (https://www.cms.gov/Medicare/CMS-Forms/CMS-Forms/Downloads/CMS116.pdf).
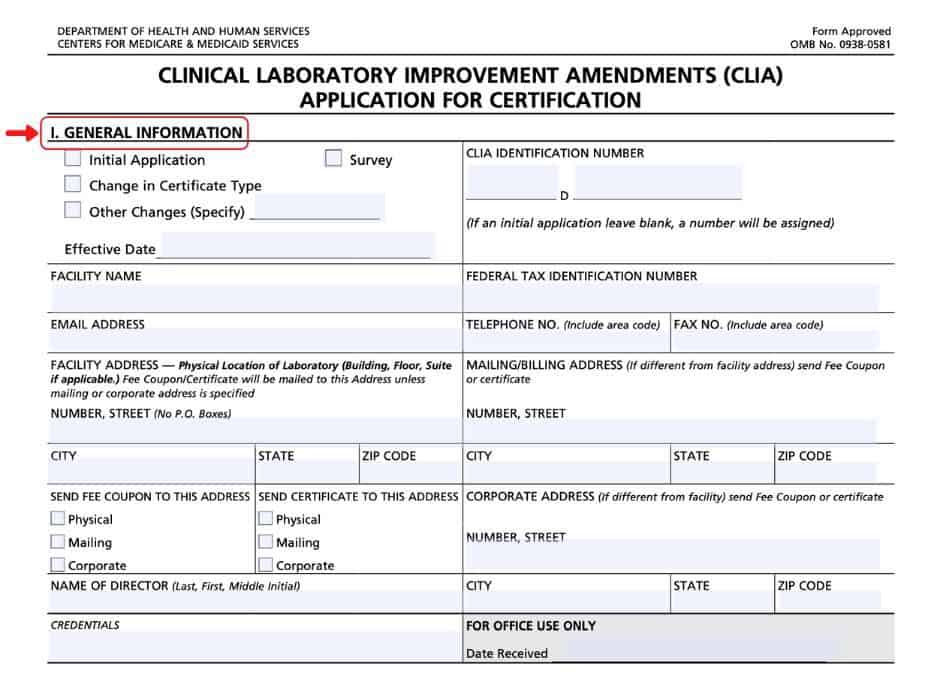
Section I
In section I of this CMS-116 form, complete the basic information regarding your laboratory and director.
Section II
In proceeding to section II, you will need to know what type of CLIA Certificate you are requesting: e.g., a Certificate of Compliance, which involves regional CLIA and state-specific oversight, OR a Certificate of Accreditation, whereby you’d be seeking accreditation through an agency such as COLA or CAP. The form contains a list of the approved Accrediting Bodies with whom you can enroll your laboratory should you choose the Certificate of Accreditation route.

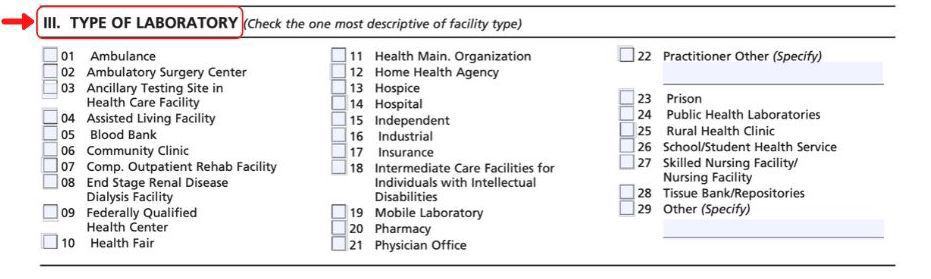
Section III
In Section III, list the type of laboratory you are starting; for example, is your facility a Physician Office Lab or an Independent Reference Lab? Mark the appropriate checkbox associated that most closely matches your facility.
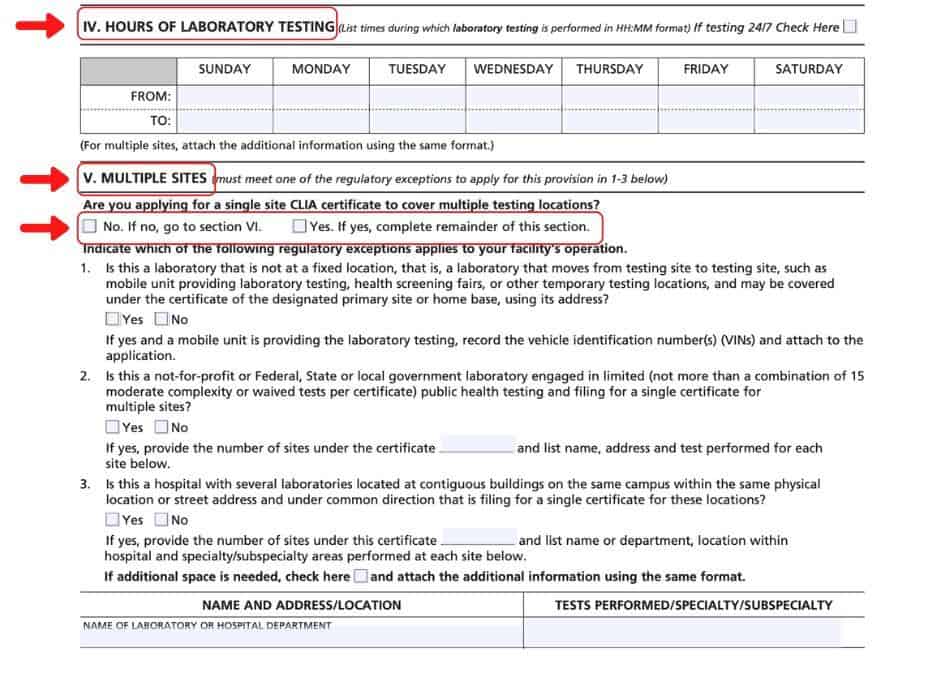
Sections IV-VII
In the next Section (IV), list the hours of the laboratory and days of the week that the facility will be open; only list the days and times that your lab will be staffed and operational. If your facility is not applying for multiple sites, waived testing, or PPM testing, please check the ‘no’ boxes that are provided in Sections V, VI, and VII, and skip ahead to Section VIII.
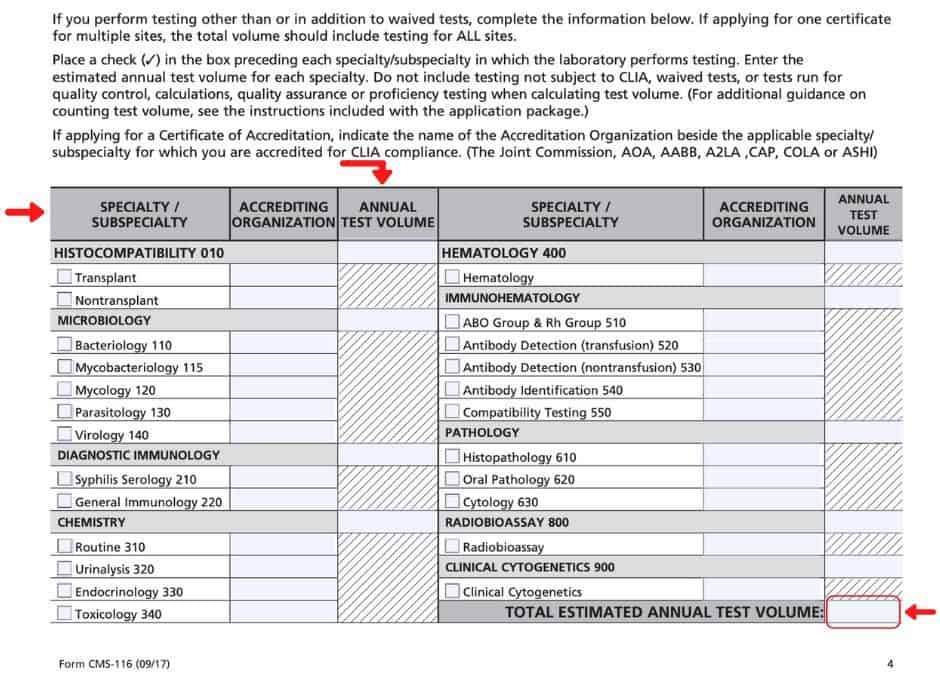
Section VIII
In Section VIII, list each analyte and device/instrument that will be used in the laboratory; for example: “Influenza A, QuantStudio 12K Flex” (analyte, device/instrument). Do this for each analyte and/or device/instrumentation that you have in your lab so that Section VIII provides a comprehensive look at your analytes and instrumentation for CLIA to review. The space that is provided can be limited, but the form includes a checkbox that should be marked if you need additional space and would like to attach more pages in order to detail all of your analytes and devices/instruments in the same format described above.

Section VIII is also where the specialty/subspecialty should be marked according to your test menu, along with the annual volume you are estimating for each specialty. If you have decided to request a Certificate of Accreditation, please list the Accrediting Organization in that same-named specific column next to each subspecialty; if you are seeking a Certificate of Compliance, leave the Accrediting Organization column blank for all specialties. Once you have estimated each specialties’ annual volume, total these numbers and enter the Total Estimated Annual Test Volume to complete Section VIII.
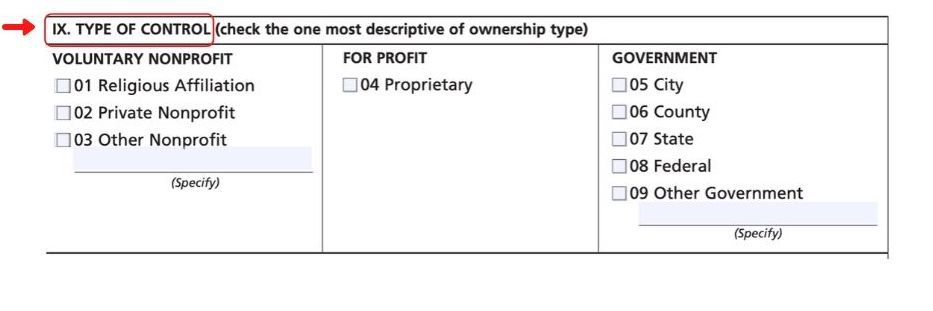
Section IX
In the next Section (IX), list the Type of Control regarding the lab’s ownership: nonprofit, for profit, or government, and check the appropriate box under the correct designation.
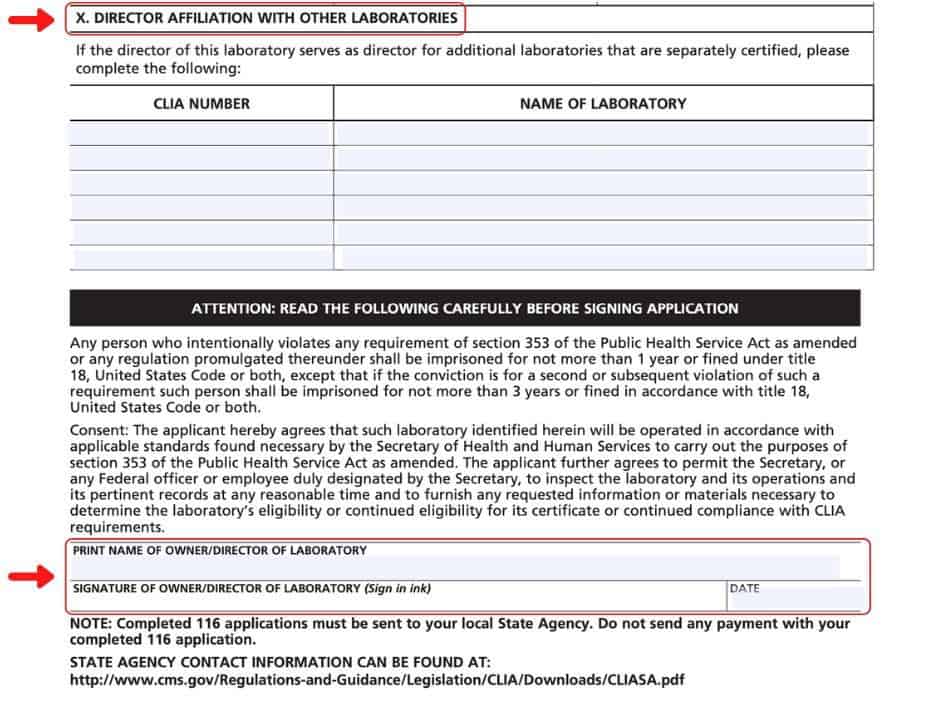
Section X
You will need your Laboratory Director to complete or provide you with his/her other CLIA Laboratory Directorships and those labs’ CLIA Numbers for section X. Typically, the application is signed and dated by the Laboratory Director, but an owner may sign it instead.
The Final Step
Now that the application is complete, it will need to be mailed to the proper State agency with the Laboratory Director’s resume, diploma/transcripts, board certifications, applicable state license(s), and the lab’s Accrediting Agency’s enrollment letter (if applicable due to seeking a Certificate of Accreditation).
We recorded a step by step video of filling out CMS-116 form process that may be more explanatory for you. You can WATCH IT HERE.
Still having trouble with the completion of the CMS-116 form? Reach out to us! We have Credentialing Specialists available to assist you that can address your questions and/or complete the application process for you!
Thank you so much
Welcome:)
Hello, we are an Intern. Med. physician we will do Ua, Covid test. Sofia 2 Quidel, Rapid test INR
PT, Ptt testing should I get a waiver ?
Hello, yes you should get a CLIA waiver. Let us know if you have any other questions.
Do we need to get a clia waiver from every state for antigen testing?
If it is a CLIA Waived test, you would need a waiver in every state that the test is being read. If they are collected and shipped to a central location, you would only need a waiver for that location. Let us know if you have any other questions:)
Hi,
This website is great. Thank you. If I want to simply set up a pop up testing center where I administer waived antigen swab tests, do I need to obtain a certificate of waiver (and lab registration in CA) if I’ll be sending the tests to a lab for reading results? If not, can I just get started administering?
Hi Dan and thank you! I believe that in CA (assuming you are not performing the waived tests at the site but collecting specimens) you need a state specimen collection license in CA. However, if you are collecting specimens and running the specimen on the test at the site then you need a CLIA waiver and state lab registration. Also, we found this link helpful for it: https://www.cdph.ca.gov/Programs/OSPHLD/LFS/Pages/COVID-19Guidance.aspx
To open a covid testing site do you require a Clia if i am collecting the specimens and sending it to a certified lab to run the samples
Sean, you don’t need a CLIA for collection, but you may need a collection license depending on the state.
Great Information Here! Thank you.
I have some questions:
I do run the courier for different laboratories that perform COVID testing, BUT o have found 3 churches where they will allow me to do COVID testing in tents. I would like to collect those COVID’s with my courier company name and send out the samples to one of the labs I work for and be able to get some extra money.
What would I need to do, or license to get that done? I’m in Florida
Thank you so much
Thanks, German! Glad you enjoyed our article and found some value in it. I’ve forwarded your question to our consulting team and will follow up with a response once they’ve had a chance to review it.
For a Change of Ownership for a CLIA Waiver, does Section VI require completion?
Hello Brenda, this depends on the state the laboratory is located in. However, we recommend that the entire application be completed. In what state the lab is located?
I have a few questions regarding completing Section VIII of CMS-116:
If we are a Mohs micrographic surgery practice and do only H&E and immunohistochemistry, do we list out “Hematoxylin” and “Eosin” separately under the “Analyte/Test” column even though those two things are run together during the processing of each slide? Do we need to list each component of an immunohistochemistry protocol as an “analyte/test” since these are multi-step protocols?
Also, how do we know which of these is defined as “M” (moderate complexity) or “H” (high complexity)?
Lastly, You mention “instrumentation” needs to be listed in this section, but since neither a microscope nor a cryostat fall under the category of “Analyte/Test” where would we list them?
Hello Charles, H&E can be listed as one test but provide as much detail as possible. You can find the complexity of the test by searching the FDA website (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCLIA/search.cfm).
Also, we know that this requires a board-certified pathologist and usually is a high complexity test.
If you need more assistance, please feel free to submit a contact form to get in touch with our team for consulting or application help (https://www.lighthouselabservices.com/contact-form/).
Actually, board-certified dermatologists are capable and allowed to read their own histopathology. FYI, and per the CPT guidelines, any physician who uses/bills the Mohs codes MUST be both the surgeon and the pathologist (i.e. they must be the same person). Anything else is not considered Mohs surgery. Thus, the dermatologist is both the surgeon and pathologist.
This is because Dermatology training includes extensive training in dermatopathology.
Are there any specific building/office space requirements to obtain a CLIA certification in order to run PCR analysis for COVID-19? We are hoping to utilize our current space/transition from only a CLIA waived lab to CLIA certified.
Thanks for reaching out to us, Samantha. I’ve forwarded your questions to our consulting team for follow up. Please keep in mind that it may take a few business days for someone to respond.
Hi!
We are a CLIA waived mobile lab, and we just want to clarify what states we can cover under our Illinois waiver.
Thanks for your question, Mackenzee. Let me check with our team and I will get back to you soon with an answer!
I followed up with our Quality Team and was informed they typically have to reach out to states on a case-by-case basis due to a number of variable factors that could impact the answer. A few of those factors include: type of testing (COVID, non-COVID, matrix), state of home base, where QA/QC documents are stored, how long the mobile unit will be at each site, whether the testing is occurring within the unit or at a pop up site or in a building and the equipment is being transported in the “mobile” unit.
While I apologize we can’t provide a direct answer in this forum, we’d be happy to investigate if you’d like to discuss a limited consulting arrangement. Feel free to reach out to info@lighthouselabservices.com if that is something you would be interested in discussing. Thanks again for reaching out!
Is the owners of the facility required on the CMS-116 form or can it just be the Lab Director? Can I leave owner of laboratory empty?
Thanks for your question, Brian. I’ve reached out to our Quality team and will let you know what they say!
Brian, our team recommends having both signatures for “just in case” reasons because some states prefer it. If there are multiple owners, only one needs to sign in addition to the Lab Director. Hope this helps!
Hello! Thank you for this very helpful article. Can the laboratory owner be the name of the entity that owns the laboratory, or does it need to be an individual person’s name?
Mary, we’re glad you found this article to be informative! I’ve reached out to our Quality team with your question and will reply here when we have a response.
Hello,
hope all is well. The procedures that we’re performing currently are phlebotomy, intramuscular injections, and wound suturing. We will be doing diagnostic imaging tests such as X-ray and ultrasound imaging in the near future.
I’m not quite sure what are the things i have to enter in section “IV . Waived Testing “, Will you please help me out with this.
Hi Nihal, thanks for your question! I’ve forwarded it to our quality team for a response. Please keep in mind their response may be delayed due to the Holidays. If you want to reach out to our broader team in the meantime, feel free to email info@lighthouselabservices.com.
how can i find out what we are CLIA waived for? For the state of Texas.
Thanks for your question! I’ve reached out to our team and will respond here once they get back to me.