Last year, we notified drug testing providers CMS was reviewing definitive and presumptive drug screening claims for errors and missing documentation as part of its Targeted Probe and Educate (TPE) program. Results for the WPS Region were published last week, showing more than 50% of the claims reviewed for CPTs 80307 and G0483 failed to include proper documentation or physician orders.
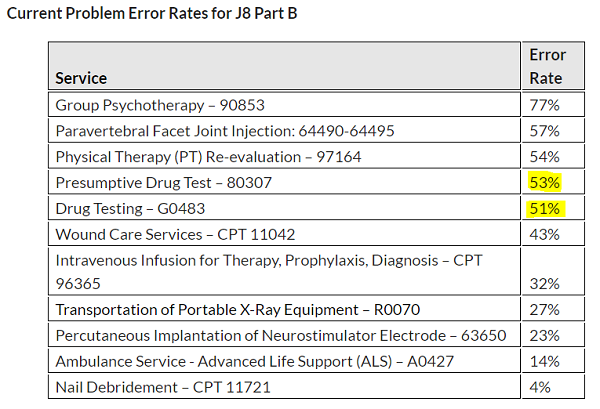
Top reasons listed for denials related to definitive and presumptive drug testing primarily relate to medical documentation either being incomplete or not supporting medical necessity requirements.
As part of this initiative, you may be selected for an audit if you’re billing these services at a rate higher than the national average. If flagged for review, you could face penalties that could significantly impact your reimbursements, such as pre-payment reviews or takeback requests.
Prepare and Maintain Billing Compliance With Our Audit Checklist
The following checklist offers a high-level overview of the medical documentation that may be requested if your laboratory is selected by CMS for a Targeted Probe and Educate Audit. As stated by CMS, this initiative aims to improve targeted providers’ overall billing compliance before engaging in punitive actions, such as takebacks or pre-payment reviews.
You can check out the full report on TPE results from WPS here with a summary of all codes targeted and reasons for the error rates. If you have concerns about these findings or would like more information, don’t hesitate to reach out to us directly to connect with our RCM Solutions Team for a free consultation.