As the COVID-19 pandemic continues, we have multiple inquires per day from individuals contemplating starting a COVID-19 testing lab or considering adding this line of testing to their existing lab. Outlined below are the most frequently asked questions.
What are other labs doing about COVID-19?
We recently polled 50+ labs to determine if they had started COVID testing or if they were pursuing it. Approximately two-thirds of labs were referencing it out, while 5.6% had validated the testing in-house and 27.8% were in the process of validating it. Also, 28.6% of labs indicated that they were considering offering a drive-thru collection of COVID-19 samples.
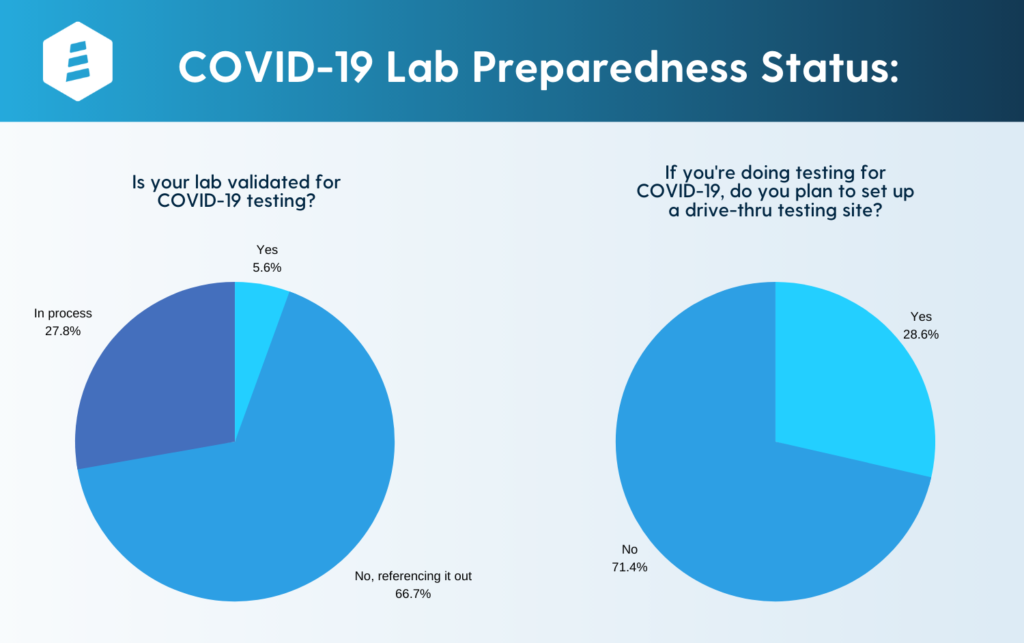 />
/>
What is the reimbursement for COVID-19 testing?
The Centers for Medicare and Medicaid Services has established two Healthcare Common Procedure Coding System (HCPCS) codes for coronavirus testing. HCPCS code U0001 is for CDC labs to use, and HCPCS code U0002 is for non-CDC labs to use when reporting SARS-CoV-2 testing. Recently Medicare said that it will pay a higher payment of $100 for COVID-19 clinical diagnostic lab tests making use of high-throughput technologies effective on 4/14/20. To qualify a lab needs to be able to test 200+ tests per day. More details here.
What equipment do we need to perform the testing and what does it cost?
There are now 45 different IVD testing options for COVID-19 that have received EUA approval. Click here for the full list. Additionally, a lab can use an open system to validate COVID-19 testing as an LDT (Laboratory Developed Test). While using an EUA approved options would seem to make the most sense there are significant delays due to waiting lists for most of these. Validating the test an LDT allows the lab to be more creative and potentially avoid the long waiting lists, however, this requires that the test be performed in a High-Complexity lab, which triggers more rigorous qualifications for the staff in the lab. Click here for a link to the staffing requirements for Moderate and High Complexity labs by state. The cost of the equipment varies depending on the vendor, throughput requirements, and automation. Also, there can be significant savings by using refurbished equipment. New high-throughput equipment with automation can cost as much $300K, however, we have set up labs with refurbished moderate-throughput platforms for as little as $45K.
What is the cost to run a COVID-19 test?
Again, this varies drastically based on the setup. However, the consumable cost ranges from $25-35/sample for most open system molecular RT-PCR systems that we validate.
What is the cost to validate a COVID-19 Molecular PCR LDT in our lab?
We charge between $19,500 to validate any Infectious Disease panel (including COVID-19) as an LDT, however, we only charge $9,500 and for each additional. Click here to read more about our validation service and request a quote.
What about antibody (serology) testing?
Many people see this as an important tool in the battle against COVID, however, the application and reliability of the results are still being debated. Common questions include: Does the presence of antibodies indicate immunity to future infection? Is the lack of presence of antibodies indicative of a negative diagnosis? Are the rapid kits reliable enough to make widely available? While we don’t currently have answers to many of these questions on 4/17 the FDA announced the following in an effort to give some clarity on the efficacy of the testing (full announcement here):
“The U.S. Food and Drug Administration (FDA) recommends that health care providers continue to use serological tests intended to detect antibodies to SARS-CoV-2 to help identify people who may have been exposed to the SARS-CoV-2 virus or have recovered from the COVID-19 infection. Health care providers should also be aware of the limitations of these tests and the risks to patients and the community if the test results are used as the sole basis to diagnose COVID-19.
The FDA is not aware of an antibody test that has been validated for the diagnosis of SARS-CoV-2 infection. While the FDA remains open to receiving submissions for these tests for such uses, based on the underlying scientific principles of antibody tests, the FDA does not expect that an antibody test can be shown to definitively diagnose or exclude SARS-CoV-2 infection.”
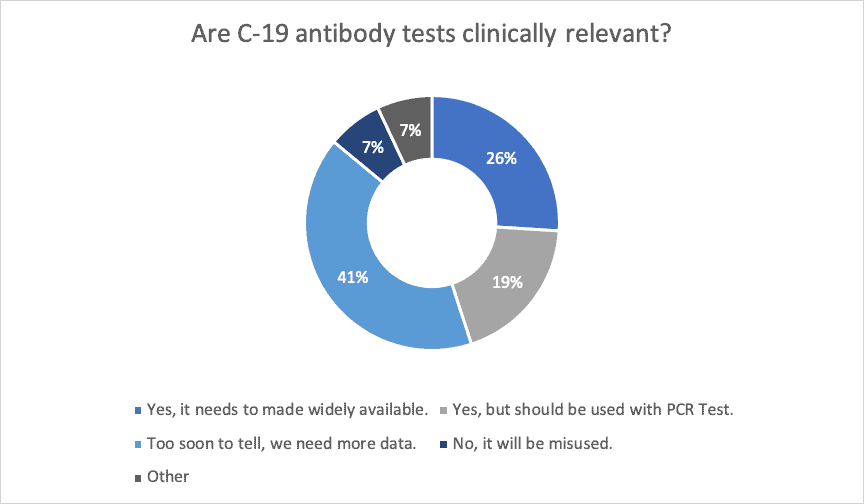
Many labs are still proceeding with procuring antibody tests. However, as these are not FDA approved and only one kit currently has an emergency use authorization (EUA), validation must meet high-complexity testing requirements. Many of the labs are experiencing trouble with sourcing controls and the positive comparison samples needed to complete the validation of the antibody test. The results of our recent poll of lab professionals on this topic are below.
Jon Harol
President
Lighthouse Lab Services
www.lighthouselabservices.com
(800) 838-0602

Have you begun selling your Covid 19 antibody test kit?
Yes, we have an offering antibody test kits and test kit validation. Please contact us for more information or visit this page on our website: https://www.lighthouselabservices.com/infectious-disease/
Great article and something needed! Great work LLS.
Thank you, Dustin!