2024 Update: Experts Weigh in on FDA Oversight of Laboratory Developed Tests
After years gestating in Congress, the VALID Act may soon finally be set to receive a vote this fall after recently being added to a broader FDA legislative package.
But despite some support from the lab industry, the act, which would create a new risk-based framework for regulating lab-developed tests (LDTs), has drawn considerable criticism from several lab advocacy groups, including the National Independent Laboratory Association (NILA), American Association of Clinical Chemistry (AACC), and the American Society for Clinical Pathology (ASCP), to name a few.
Chiefly, these organizations and others opposed to the Verifying Accurate, Leading-edge IVCT Development (VALID) Act understand that while the intent of ensuring safe and reliable testing is commendable, the regulatory framework it would create may prove too burdensome and erect significant barriers for labs providing innovative tests to patients in a timely manner.
Meanwhile, advocates of the bill contend the current regulatory framework for LDTs, in which CLIA and state regulatory bodies ensure testing quality but not the clinical validity of LDTs, does not do enough to ensure labs are providing correct diagnoses for the diseases and disorders they claim to detect.
The new “in vitro clinical tests” category created under VALID would apply to both commercial test kits, as well as LDTs. The act would create requirements for high-risk tests to receive premarket review, while lower-risk tests could reach market through a less arduous “technological certification.” LDTs currently in use would be “grandfathered” in under new rules.

Jon Harol
Jon Harol, President of Lighthouse Lab Services, a North Carolina-based laboratory consulting and recruitment firm that specializes in lab buildouts, says the current LDT framework grants labs the flexibility to respond to supply chain issues while at the same time being innovative enough to meet market demand, both benefits he believes would be eliminated if VALID were to pass.
“VALID adds hurdles and bureaucracy to new laboratory testing coming to market,” Harol says. “It slows the process down and makes it more expensive, especially for smaller labs.”
Concerns from NILA and medical laboratory professionals
For Dr. Varsha Meghnani, PhD, MB(ASCP), TC(NRCC), a technical consultant for Lighthouse who’s served as a lab director for high-complexity CLIA labs since 2017, it’s clear the intention behind VALID is to protect public health by ensuring patients are receiving quality lab tests. However, she says she’s concerned the act in its current form may have an overall adverse effect by hampering innovation.

Dr. Varsha Meghnani
“Novel tests, developed by individual labs for conditions when no commercial test is available, or their currently-available test doesn’t meet the current clinical needs, are very important in diagnosing and treating patients,” Meghnani says. “Due to a slowing down in innovation, patients might lose access to these tests.”
She also expressed concerns with the burden the test registration process could have on small, short-staffed labs or academic labs with limited funding.
NILA, meanwhile, wrote in a May 21 letter addressed to the chairs of Senate Health, Education, Labor & Pensions (HELP) Committee that LDTs serve an “irreplaceable part in patient care” due to their ability to be developed rapidly in response to new threats to public health.
“LDTs are frequently more accurate, reliable, and relevant to patient care than FDA-approved IVDs,” NILA wrote. “Because LDTs are regulated by CLIA and do not require FDA approval, laboratories are able to modify existing LDTs safely and accurately to improve their performance, meet the needs of patients, and respond to emerging threats.”
Impact to toxicology
Another concern with VALID is the severe impact it could have on the toxicology industry’s ability to develop tests that can detect rapidly evolving designer street drugs.
Oftentimes, new drugs can take significant time to be detected by law enforcement. And once that occurs, toxicology labs must move swiftly to create LDTs that can detect those drugs.
As Harol points out, essentially every existing toxicology confirmation method is currently an LDT.
“If labs have to go through an FDA process to get a test approved every time a new drug pops up, it could extend the window of illegal drugs being on the market for years,” Harol says.
Support from others
As previously mentioned, while there is a significant amount of opposition to the act in its current form, it does have some high-profile supporters, including the College of American Pathologists (CAP).
Essentially, while CAP acknowledges the concerns other groups and lab professionals have about the regulatory burden VALID would create, the organization contends it would rather support and provide input on VALID instead of tossing it out altogether given that the bill’s bipartisan support and bicameral status shows it’s likely to pass regardless of opposition.
In a May 26 message from CAP President Emily Volk, MD, FCAP, Volk outlines that while many in the medical lab industry feel the regulations created under VALID go too far, some patient advocacy and cancer groups believe they don’t go far enough.
“In the current form, the VALID Act contains many provisions that are similar to policy the CAP has advocated for regarding the regulation of laboratory tests since 2009,” Volk says in her letter. “Importantly, the current version includes explicit protections for pathologists and our ability to practice medicine without infringement from the Food and Drug Administration (FDA).
 “No legislation is perfect”, She continues. “However, the latest version of the VALID Act includes provisions that will allow us to meaningfully influence the enforcement and execution of the legislation through the regulatory process.”
“No legislation is perfect”, She continues. “However, the latest version of the VALID Act includes provisions that will allow us to meaningfully influence the enforcement and execution of the legislation through the regulatory process.”
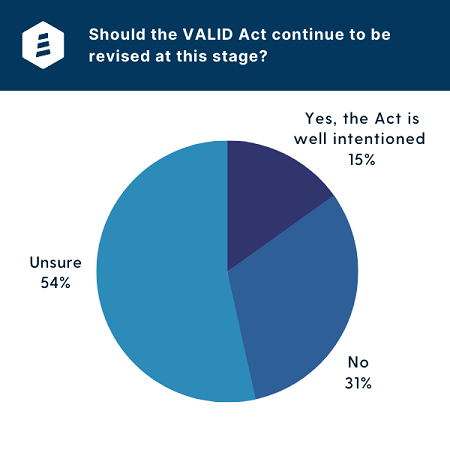
During a June 28 webinar on the status of VALID hosted by Lighthouse, 86 lab owners and decision makers responded to a poll asking if they feel the VALID Act should continue to be revised at this stage. Of those, 85% answered “no” or indicated they would need to review any potential changes before the act could gain their support, while the remaining 15% stated they would like to see the bill’s eventual passage due to the need for additional LDT oversight.
Erin Morton, Senior Vice President for CRD Associates, a D.C.-based lobbying firm working on behalf of NILA to raise awareness of the issues VALID could create, encouraged listeners on the webinar to reach out to their member organizations and their leadership if they have questions or concerns about their support for VALID.
Potential amendments and actions steps
While Meghnani says she doesn’t support VALID in its current form, it could still be amended to be less burdensome for her fellow lab directors.
Primarily, Meghnani says the FDA should direct its focus mostly on high-risk IVCTs and direct-to-consumer tests where physicians are not directly involved in interpretation and treatment decisions. Secondly, she believes commercial manufacturers of LDTs should be made responsible for establishing the clinical utility of their tests, as opposed to the individual small labs who utilize the kit.
“Lastly, the registration process should be made very user-friendly so that small labs are not overly burdened,” Meghnani concludes.
The organizations advocating against VALID are now working to get the act removed from being packaged with FDA Medical Device User Fee Amendments – which require all companies putting a product through FDA review to pay a user fee – that must be passed prior to a September 2022 expiration. These fees account for more than half of the FDA’s annual budget and would likely lead to VALID being passed without significant discussion if still attached at the time of the vote.
To support this effort, NILA is strongly encouraging its members and other interested groups and individuals to reach out to their local representatives to express their concerns with the VALID Act. Specifically, those who do so should be prepared to express how VALID could negatively impact their lab and patient population.
Additionally, the Association for Molecular Pathology continues to seek signatories for its letter outlining its specific concerns with VALID. Those interested may review and sign on here.

Basically I am against government to control any analytical or diagnostic testing as they can use this for their political purposes.