Clinical laboratories and healthcare providers as a whole received a number of welcome reprieves from potential payment cuts and burdensome regulation as 2022 drew to a close. But that doesn’t mean many of these issues couldn’t be resurrected in a different form in 2023.
From the ongoing workforce shortage to the demise of the VALID act, here are six recent developments we’ll be keeping an eye on this year:
PAMA delay, clock starts again on another long-term fix
While it wasn’t the long-term solution many in the clinical lab community had hoped for, labs were able to breathe a sigh of relief after a one-year delay for substantial payment cuts of up to 15% for roughly 800 tests on the Clinical Lab Fee Schedule (CLFS). Additionally, the next round of private payer data reporting many labs would have been required to submit during the first quarter of 2023 has also been delayed for another year.
The data reporting period has now been delayed for a third consecutive year, something most lab advocacy groups have applauded as many contend the current data collection process relies too heavily on rates submitted by large reference laboratories.
Despite these wins, PAMA cuts and reporting are set to resume in 2024 unless a legislative fix can be passed before that time. While many lab advocates rallied around the Saving Access to Lab Services Act (SALSA), which would have capped annual rate reductions and sought to draw data from a more statistically representative sample of the entire clinical lab market, the bill ultimately was not included in the year-end omnibus funding package. Now, it remains to be seen if SALSA or another fix will be passed in 2023.
VALID did not advance. What’s next for FDA regulation?
The VALID Act, which would have created a new risk-based framework for regulating lab developed tests (LDTs), was excluded from the year-end omnibus spending package, meaning the legislation won’t advance at this time.
Lighthouse and several lab advocacy groups have long advocated against the Verifying Accurate, Leading-edge IVCT Development (VALID) Act, contending that while its intentions were good, the regulatory framework it would create by granting the FDA oversight of LDTs would prove too burdensome and erect significant barriers for labs providing innovative tests to patients in a timely manner.
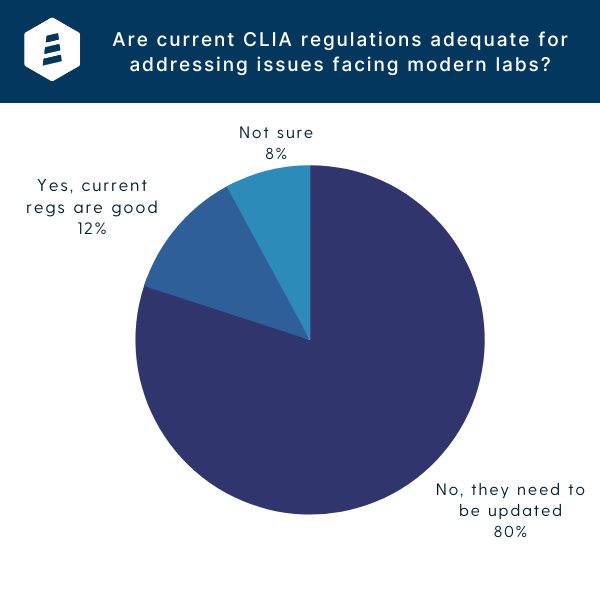
Recent LinkedIn poll of 290 clinical laboratory stakeholders.
However, as Lighthouse President Jon Harol points out, current CLIA policies and regulations have not been amended for roughly 20 years and may soon require some sort of overhaul to address issues modern labs are facing with new technologies such as algorithm-based tests and artificial intelligence.
That change could come in the form of rulemaking by the FDA, which has largely signaled the agency is still interested in heightened LDT oversight in some form. Additionally, several private payers have begun to impose their own accuracy requirements that extend beyond what labs are required to show for CLIA or CAP, accreditation, according to reporting from 360Dx.
Whether it happens through a revised form of VALID or another fix altogether, it’s clear regulatory change for LDT oversight and overall CLIA policies may soon be on the horizon. In a recent poll of 290 clinical lab stakeholders conducted on LinkedIn, 80% of respondents said they believe it’s time to update CLIA regulations to adequately address issues modern labs are facing.
CLIA applications for new labs declining

Jon Harol, President, LLS
According to data reviewed from Lab Prospectus, Harol calculates new CLIA lab applications declined by about 63% in December 2022 when compared against the monthly average in 2022. While this may raise a red flag to some over concerns about patient access, Harol is cautiously optimistic that this downturn in new labs represents a market correction from the explosion of new competition spurred by the COVID pandemic.
“The industry was never going to be able to continue to grow at the rate it did when there was the need for regular and widespread COVID testing,” Harol points out. “But it remains to be seen exactly what this decline in new labs will mean for the future of the industry and competition amongst existing labs.”
X-Waiver/MAT Act news (now no restrictions on doctor-to-patient ratio, but new training burdens imposed)
In a win for toxicology labs and providers, the spending package included the Mainstream Addiction Treatment (MAT) Act, which removed X-waiver limits on the number of patients practitioners are allowed to treat. The elimination of the waiver also removed waiver requirements for providers seeking to prescribe buprenorphine as a medication-assisted treatment for substance use disorder. Since a waiver was not required to use the drug to treat pain, many have applauded this move as removing an unnecessary burden.
However, the package did introduce a new regulatory burden through the Medication Access and Training Expansion (MATE) Act, which will require healthcare providers applying for or renewing a DEA narcotics prescribing license to complete an eight-hour course on treating addiction. This course will be required in addition to required coursework already mandated by several states and could in turn lead to an overall decrease in the number of providers treating pain and addiction.
MolDX still not approving technical assessments
While the MolDX program has been governing utilization of molecular and genetic testing in several MACs for several years, 2022 was when many labs first encountered reimbursement challenges related to infectious disease testing due to the introduction of new policies and coverage determinations. Chiefly, the program now requires the technical review of any infectious disease panels, including FDA-cleared tests with five or more targets.
The catch, however, is that the “technical assessment” process has been notoriously opaque and has created a moving target as to what clinical studies would be sufficient to satisfy utility and validity requirements to receive a fixed reimbursement rate. MolDX representatives have said they are seeking peer reviewed articles demonstrating clinical utility in terms of improved patient outcomes, although only STI panels have received approval at this stage.
- RELATED ARTICLE: What is MolDX and When Do I Need a Z-code?
Given the length of the review process (roughly two months) and confusion surrounding acceptance criteria being sought, Lighthouse is working to gather interested labs as part of a coalition to share studies and develop a better understanding of how to best navigate MolDx requirements to ensure consistent reimbursement.
“I do feel there’s a need for laboratories to work together on this issue,” Harol said during a recent webinar comparing PCR and culture testing. “There’s a heavy lift for any one laboratory to put together the documentation and research that’s needed to respond appropriately.”
Continued lab workforce shortage
While the shortage of medical laboratory professionals isn’t a new development, it’s certainly one labs will continue to contend with as they seek to combat burnout and industry-wide understaffing. A recent report by Forbes estimates the US currently has a shortage of about 20,000-25,000 medical technologists. The issue is further exacerbated by the fact there has been a 7% decrease in the total number of Med Tech and Medical Lab Scientist training programs since 2000.

Maggie Morrissey, Director of Recruiting and Staffing, LLS
Last year, Lighthouse conducted its first wage and morale survey of medical lab professionals and found that more than 1,100 respondents were largely feeling overworked and underappreciated when compared to their peers in other healthcare sectors. While some of those morale issues can be addressed through improving workplace culture and communication, many frustrations due to staffing limitations or retention struggles will only be solved through long-term advocacy for the profession.
Maggie Morrissey, Director of Recruiting and Staffing for Lighthouse, predicts staffing concerns will grow worse in the coming years before developments like automation and recent efforts to raise awareness of the need for new med techs bear fruit.
“I do think the staffing issue is unfortunately going to get worse over the next three to five years,” Morrissey says. “Maintaining a strong culture with competitive compensation and benefits will be crucial for retaining top talent in the years to come.”
If you have questions about how any of these issues will impact your lab’s day-to-day operations or overall revenue cycle, feel free to reach out to us to discuss them further during a free consultation.
