Information provided by Tami Shaw, Client Manager, Lighthouse Lab Services
Palmetto and WPS delayed enforcement of the LCD highlighted in this article until May 17, 2022.
The LCD highlighted in this article went into effect for CGS and Noridian on June 2, 2022.
Laboratories performing infectious disease testing and billing Medicare Part B within a MolDX jurisdiction are subject to a new Local Coverage Determination (LCD) that stands to significantly impact reimbursement for these services.
Labs in the impacted MACs (Noridian, Palmetto, CGS, and WPS) are required as of April 17, 2022, to register for a unique DEX code z-identifier through the Diagnostic Exchange to receive a fixed reimbursement for laboratory developed tests (LDTs) or “expanded” panels that test for more than five targets.
Read on below to learn more about the history of the MolDX program, the purpose of Z-Codes, and the process labs need to take to complete this registration in order to continue being able to bill for impacted tests.
The history and purpose of MolDX
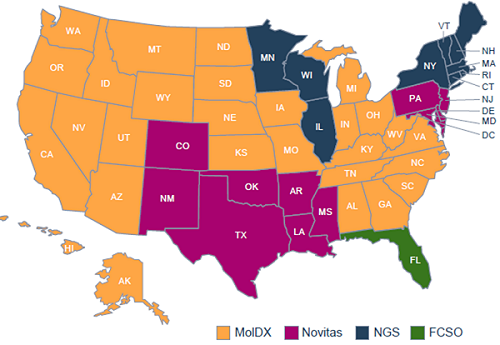
Current states participating in the MolDX Program
The Molecular Diagnostic Services (MolDX) Program was developed by Palmetto GBA in 2011 to identify and establish coverage reimbursement for molecular diagnostic tests. This program currently provides uniform policies for 28 states, across four Medicare Administrative Contractors (MACs).
MolDX defines a consolidated set of LCDs, articles, and coding edits. The other participating MACs operate using the MolDX program based on joint agreements. The program primarily performs the following functions:
- Facilitates detailed and unique identification through registration of molecular diagnostic tests to facilitate claims processing and to track utilization.
- Establishes clinical utility expectations.
- Completes technical assessments of published test data to determine clinical utility and coverage.
- Establishes reimbursement rates.
What’s a DEX Z-Code?
The Diagnostic Exchange (DEX) Z-Code Identifier is a unique five-character alpha-numeric code associated with certain molecular diagnostics tests and is used by some payers as an adjunct to non-specific CPT codes. This code is unique to a lab’s specific molecular diagnostic test (MDT), or LDT.
This code, submitted on a claim, in addition to the CPT/HCPCS code, provides clarity to help ensure that both payers and providers clearly understand which test is being ordered, performed, and billed.
When do I need to obtain a Z-Code?
Laboratories seeking coverage for the following types of tests must obtain a test ID:
- LDT or MDT reported using an unlisted code
- Test reported with a Tier 1 or Tier 2 CPT code
- FDA-approved version of an MDT test (if multiple, identical versions of the test are available, including tests that have not been approved by the FDA)
- All versions of a single test performed in multiple laboratories (to the extent that each laboratory performs the test differently)
- Modified version of an FDA-approved IVD
How does reimbursement for infectious disease testing stand to be impacted by this new LCD?
Effective April 17, infectious disease panels performed and billed in MolDX jurisdictions are subject to a new LCD: Molecular Syndromic Panels for Infectious Disease Pathogen Identification Testing.
Prior to the introduction of this LCD, ID panels testing for more than five pathogens were not reimbursed for any reason. Only three to five pathogens were considered medically necessary.
The expanded panels listed in this LCD are:
- Respiratory panels
- Pneumonia panels
- Gastrointestinal panels
- Urogenital panels
- Anogenital panels
- Meningoencephalitis panels
- Bloodstream Infection panels
- Urinary Tract Infection panels
The panels not mentioned in this LCD, but that are affected by it, could include:
- Sexually Transmitted Infection panels
- Women’s Health panel
- Wound panels
- Nail and Fungal panels
Expanded panels will now require an ID specialist to order. There are exceptions for rural and remote areas where access to an ID specialist is unreasonable. In general, the exception would require that the ordering provider is located closer to the patient’s residence than the nearest specialist.
Some of the guidance on ID specialists includes:
- For Immune-competent beneficiaries, must be ordered by:
- Pulmonologist for respiratory and pneumonia panels who is treating the patient.
- Gastroenterologist for GI panels who is treating the patient.
- For Immune-compromised beneficiaries
- Must be ordered by a Clinician Specialist
- Infectious Disease (Any Panel) Must be treating patient.
- Oncology (Any Panel) Must be treating patient.
- Transplant (Any Panel) Must be treating patient.
- Pulmonologist (for Respiratory and Pneumonia panels) Must be treating patient.
- Must be ordered by a Clinician Specialist
Panels that continue to target three to five pathogens will not require an ID specialist.
What steps should our lab be taking now if we haven’t already registered my tests?
Most ID panels, including RPP and GI expanded panels (more than five pathogens) will need to be registered with the MolDX and have a DEX Z-code assigned to them, which will create established pricing. If the lab has not already registered with the MolDX program, there are three primary steps:
- Register with MolDX program. Each lab can have two representatives assigned access.
- Register the tests.
- Submit a Technical Assessment.
This process can take approximately 60 to 90 days to complete.
While it may require a fair amount of front-end work, there are benefits to receiving MolDX Z-Codes. Receiving a fixed rate for your tests allows labs to create a consistent pricing model, even if reimbursement in some instances is lower. This will also reduce denials, similar to a preauthorization process. In short, your staff or billing team will face fewer hurdles to get reimbursed for your services.
If you need assistance with registering for a DEX Z-Code, completing a technical assessment, or any other step in this process, Lighthouse has the technical and RCM expertise to guide you and ensure you continue receiving the maximum reimbursement when billing Medicare for these services.
Schedule a free consultation with us today!
Is this for the MolDX states in the above map in orange. We are Novitas (Louisiana) so are we included in this new policy? If not is this going to be implemented for us at some point in time?. Thank you for your help.
We do file to Palmetto for RR Medicare pts. So would Louisiana be included in this new requirement?
Hi Serenna,
Thanks for your questions. Novitas is not included in MolDX at this time and there’s currently no timeline as for when/if they may join. I will follow up with our RCM team on your question about RR Medicare and get back to you soon with a reply.
Is Novitas still excluded in MolDX (2023)?
That’s correct. Novitas, NGS and First Coast are not part of MolDX currently.
Fee for service Medicare in Novatias may be excluded from MolDx but FYI, UHC Medicare Advantage requires it nation wide. UHC is rolling out the MolDx requirement in “waves” by CPT code:
https://www.uhcprovider.com/content/dam/provider/docs/public/health-plans/medicare/2021/MedAdv-Z-Codes-Palmetto-FAQ.pdf
Great point, Sarah. Thanks for adding this note!
Is MoIDX eligble forFLorida State ?
Hi Anish, Florida is in the First Coast MAC, which is not currently part of the MolDX program.
For Kentucky, is part of CGS, which this applies to. So will an independent lab just not get paid on these if they do not register with the program or is it that they just will not know what reimbursement is assigned to them?
Typically, you should expect to see denials for these services if you haven’t registered.
So if they are not getting denials and receiving payment, what would your advise be if they do not want to register for the Moidx?
That would be a situation we would need more information from you on in order to determine whether it’s a compliant billing arrangement. We’d be happy to discuss the issue further in an initial free consultation if you reach out to us at info@lighthouselabservices.com with some basic info.
If a lab summitted the Z-code amplification, got an Z-code, but there is no assigned coverage (not covered), does that mean this service will be denied by Medicare and there is no way to do a case-by-case appeal?
Hi Zain,
Apologies for the delayed reply, a lot of our team was off for the holidays and we’re just catching up on a number of these questions. Here’s the reply from our RCM team, don’t hesitate to let us know if you have additional questions!
Reply: Yes, you are correct. If a lab has not yet received a CPT Code from MolDX along with a defined reimbursement, the lab should anticipate seeing those services denied by Medicare. Because the panel being performed is not already FDA approved, MolDX requires that the clinical utility and validity of the panel be proven through a Technical Assessment process. Until that time, the panel is considered to not be medically necessary. There is no method in which medical necessity for an unproven panel can be demonstrated and appeals are not seen to be helpful in these situations.
I have found that some mold x codes suggest switching the cpt code to a different cpt code so that the bill will get paid. should the original cpt code be remove and switched to the recommended code?
Hi Kelly, here’s the response from our RCM team. Let us know if you have any follow up questions!
Reply: It is only upon successful completion of the Technical Assessment that your Z Code will be matched to a new/existing CPT Code and assigned reimbursement. If you’ve gone through that process and the MolDX has provided you with the CPT code that they have approved for your panel, you would begin utilizing that defined CPT Code in combination with the associated Z code on your claims going forward. The original CPT code that you were using for that panel would no longer be applicable.
like to see if any more information available. if we have PLA code for a panel. how we set coverage determination?
guideline for TA submission. do you have template?
can you see our billing is in compliance?
Hi Leena, thanks for reaching out with these questions. We do have additional info we can share on PLA codes and coverage if you’d like to reach out to us with a bit more information about your issue by emailing info@lighthouselabservices.com. We unfortunately don’t have a general template for TA submissions since they can vary widely. And ensuring billing compliance is also an item we can assist with if you’d like to reach out to us.
The policy require Z-code and CPT code and TA if a laboratory test more than 5 pathogens. What if we can only perform most common five pathogens using UTI PCR? What Z-code and CPT codes will be used for billing that meets medical necessity and get paid by Medicare?
Hi Goverdhan, you only receive a Z-code and assigned reimbursement after registering with MolDX and completing the Technical Assessment process. During the TA it’s determined whether your test meets medical necessity requirements.
If our lab performs FDA approved MDTs such as Biofire Respiratory Panel or Biofire GI Panel, is it a requirement for us to apply for a MolDx code also since the test is FDA approved?
Hi Misha, thanks for your question. I’m following up with our RCM team and will get back to you here when I have a response.
Misha, here is the response from our RCM Consulting team. Please don’t hesitate to follow up with us at info@lighthouselabservices.com if you have additional questions.
Response: Yes, they will have to apply for a unique test identifier (Z-Code), but will not have to complete a Technical Assessment. Per the MOLDX manual:
Laboratories seeking coverage for the following types of tests must obtain a test ID:
• LDT or MDT reported using an unlisted code
• Test reported with a Tier 1 or Tier 2 CPT code
• FDA-approved version of an MDT test (if multiple, identical versions of the test are available, including tests that have not been approved by the FDA)
• All versions of a single test performed in multiple laboratories (to the extent that each laboratory performs the test differently) • Modified version of an FDA-approved IVD
Hello,
Does Toe Nails and Wound PCR require Z-codes to bill?
Hi, That will depend on a few factors, including whether you’re in a MolDX region and the number of targets. Our team would likely need to know a bit more about your specific situation, so feel free to reach out to us at info@lighthouselabservices.com. Thanks!
I’m a Laboratory biller. Doe we need to register for MoIDX program?
Hi! That will depend on whether or not your lab is located within one of the MolDX MACs and what type of tests you are performing. Feel free to reach out to us if you’d like to offer more information about your lab so we can provide a better answer.
They are some tests that include the reference labs CPT codes and then the MolDX recommends a different code. (eg….ARUP test code 3001535 = 81306 and 81335 CPT Codes, but MolDX and DEX recommends 81479)
Does this mean we use 81479 along with the ZCODE to bill in order for it to be paid?
Thanks for your question, Sherry. I’ve submitted it to our RCM Consulting Team and will respond here when they get back to me.
Hi, any response from your RCM Consulting Team?
Thanks
It looks like our follow-up on this question slipped through the cracks. I’ve resubmitted it for a response and will follow up by the end of the week. Thanks for checking with us!
Here’s what our team had to say: “When the MolDX comes back and assigns CPT codes to use, then at that point the Z code identifier + assigned CPT = the determined reimbursement. Put another way, the CPT code assigned must be used with the z-code assigned for that specific test.
August 1 is the date for Group 1 codes. Has a date for Group 2 codes been established?
Hi Summer, thanks for your question. Is this in reference to the upcoming UHC Z-Code requirements? If so, I don’t believe a date has been set yet for Group 2 codes, however, I will check with our RCM Consulting Team and get back to you here!
I have a PCR lab and have a patient that went to the ENT and we did a smallABR and RPP panel on her .
1) the RPP came back fine but it was 12 pathogens we tested for
2) we did the “small”ABR panel to determine which antibiotic was correct for her ( only six pathogens which MoldX is saying that is the max they will pay for) and they all turned up “NOT DETECTED”. She was prescribed four antibiotics for her sinus infections and none of them worked until the Dr blindly gave her a 5th and it worked. So I ran a ABRXL panel ( which is 32 pathogens) and low and behold all four she was resistant to showed up as “DETECTED”. In saying this. How am I and my Dr’s going to be able to run an ABR panel with 32 pathogens to figure out which antibiotic works for her if I am only getting paid for five??? clearly I tried to do the lower test first but it didnt give the doc the results he needed to see thus the reason we did the XL panel of 32 and we found out what was wrong with her but I dont get paid for it??? Makes absolutely zero sense……
Hi Marcus, Thanks for reaching out to us on this – we know firsthand how frustrating it can be to navigate MolDx rules when trying to provide the appropriate care a patient requires. We reached out to our RCM Solutions team directly with this question and they said it would be best for you to email us directly to schedule a free consultation so we can learn more about the specifics of your situation. You can email us directly at info@lighthouselabservices.com to request a meeting!
I sent the email requesting a call this morning. Thanks
You’re welcome. Thanks for following up with us – our team will be in touch soon!
We have a PLA code and pricing which was set by CMS. Also, claims have been processed with no issues. Our test is algorithmic protein based test. Now, it seems we need to obtain a Z code as well.
Would this change our PLA code or pricing?
Hi Missy, obtaining a Z-Code should not impact your PLA code or pricing. However, there are some nuances of Z-Code usage and the technical assessment process that you may want to be aware of prior to beginning the process if it’s your first time. If you’d like to reach out to us for more info, we’d be happy to schedule a free consultation: info@lighthouselabservices.com.
MolDx completed review of TA for PGX panel and set panel as non-coverable. what can be done here ?
Hi Nitesh, thanks for reaching out to us. Unfortunately, this is an issue we’re going to have to continue to advocate for as an industry. In the meantime, MolDx determinations are set and can’t be further appealed.
Thanks for the reply , whats the solution or your suggestion on this ?
Hi Nitesh, I can’t offer a general solution, but there may be compliant strategies you can pursue to obtain reimbursement depending on the specifics of your billing arrangements. If you’d like to schedule a free consultation with us, you can send an email to info@lighthouselabservices.com.
Hi,
How Quest bill their large panel in MolDx region? We offer the same thing!
Hi Sam,
Thanks for your question. Unfortunately, we can’t speak to why other providers may be able to bill for certain panels without seeing additional details! Let us know if you need any guidance on getting your panel reimbursed or obtaining a z-code.